The P2P program uses a structured process that takes approximately 2 to 3 years to complete. ODP provides leadership, support, funding, and coordination for the program. Partners from across NIH, other federal agencies, content-area experts, and community members help plan each workshop. We encourage others who are interested in a workshop topic to participate by attending the workshop, engaging in the discussion sessions, commenting on the draft evidence review and independent panel report, and sharing workshop resources.
Workshop planning takes place in three phases: (1) proposal review and approval, (2) planning and implementation, and (3) dissemination and follow-up.
NIH Pathways to Prevention Process
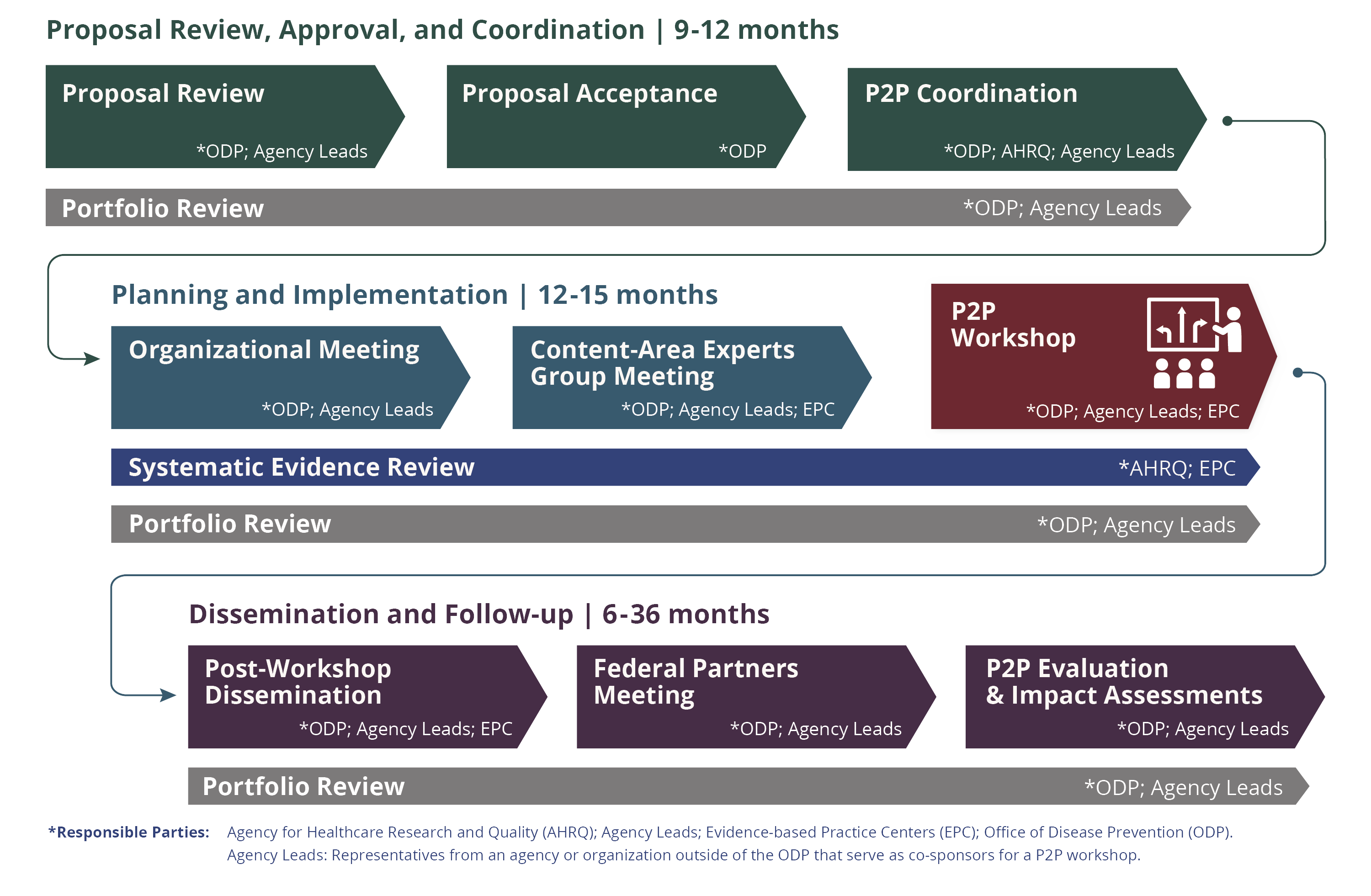
Proposal Review and Approval
Step 1: Proposal Submission
NIH Institutes, Centers, and Offices (ICOs) may submit a proposal for a P2P workshop. Other government agencies or cross-agency workgroups, professional societies, and advocacy organizations may propose topics in collaboration with an NIH ICO sponsor.
Many prevention topics may be considered for the P2P program. The following criteria must be met to qualify for a P2P workshop:
- Have a primary or secondary prevention focus
- Have broad public health importance based on the severity of the problem, feasibility of interventions, the need for a better understanding of the current research gaps, and other considerations
- Have limited published data or incomplete or underdeveloped research
- Have a need for synthesis and critical assessment of the current state of the science
To learn more about submitting a proposal, email NIHP2P@mail.nih.gov.
Step 2: ODP Review
ODP reviews workshop proposals on a rolling basis to see if they meet the P2P workshop topic criteria. Accepted proposals move to the workshop planning and implementation phase. Once a proposal is accepted, ODP initiates a review of the grant portfolio in the workshop topic area and works with the Agency for Healthcare Research and Quality (AHRQ) to commission a systematic evidence review through AHRQ’s Effective Health Care Program.
Step 3: P2P Coordination
ODP continues to meet with the Agency Leads and AHRQ to discuss the proposal, further refine the key questions, and identify federal partners. Preparations for the systematic evidence review, portfolio review of NIH funding and support for activities related to the workshop topic, and Organizational Meeting begin.
Workshop Planning and Implementation
Step 4: Organizational Meeting
A committee of federal employees reviews the scope, key questions, and timing of the workshop and nominates members of the Content-Area Experts Group and the workshop's independent panel.
Step 5: Content-Area Experts Group Meeting
Content-Area Experts Group members are experts in the workshop topic. They are a diverse group of professionals and advocates from a variety of settings, including the federal government, academia, clinical practice, and patient communities. During the Content-Area Experts Group Meeting, these experts work together to make recommendations about the workshop’s key questions, agenda, and speakers.
Step 6: P2P Workshop
The workshop includes presentations by experts on the workshop topic and the AHRQ-supported Evidence-based Practice Center that conducted the systematic evidence review. Public questions and comments are encouraged during discussion periods following each workshop session.
On the day of the workshop, AHRQ releases its draft systematic evidence review for public comment.
After assessing the systematic evidence review, expert presentations, and public comments, the independent panel writes a draft report that summarizes the workshop and identifies research gaps and future priorities.
About the Independent Panel
Each workshop’s independent panel is comprised of a select group of non-federal subject matter experts who represent diverse professional perspectives and disciplines relevant to the workshop topic. The panel gives a balanced, objective, and informed assessment of the evidence collected and presented during the P2P workshop.
The panel attends the full workshop, where they listen to and ask questions about presentations from expert speakers. The panel members then write a report that synthesizes all the information presented during the workshop and identifies research needs and gaps.
Panel members receive an honorarium for their efforts and reimbursement for travel expenses related to their participation in the P2P workshop.
While ODP staff provide administrative and technical support to workshop panels, each panel is independent. The independent panel report and recommendations do not require NIH approval and are not policy statements of NIH or the federal government.
Workshop Report and Follow-Up
Step 7: Post Workshop Dissemination
Within a few weeks of the workshop, ODP posts the draft independent panel report for public comment. Public comments are accepted for four weeks. The panel may then edit the report for clarity, correct any factual errors that might be discovered, and revise the draft report based on the public comments received.
The final independent panel report and systematic evidence review are published in a peer- reviewed journal and posted on the workshop webpage. The recommendations in the panel’s report are intended for use by the broader research community. Although NIH convenes the P2P workshops, independent panel reports are not policy statements of NIH or the federal government.
Step 8: Federal Partners Meeting
ODP brings together partners from federal agencies who work in areas related to the workshop topic to review the panel report recommendations, explore opportunities for collaboration, and outline the next steps to move the field forward. After the meeting, ODP publishes a Federal Partners Meeting Report summarizing the discussion and proposed action plan.
Step 9: Evaluation and Impact Assessment
To better understand the effectiveness of the P2P program, ODP evaluates the program’s processes, implementation, and impact such as:
- Publication of new funding initiatives
- Increased collaboration among the federal partners on the P2P topic
- Implementation of recommendations from the panel’s final report and the federal partners’ action plan
- Citations of workshop publications.
The results of this evaluation help guide decisions about the program and future workshops.